A Thaliana Defense Genes Agains Oomycetes
Introduction
Oomycetes represent a grouping of eukaryotic microorganisms related to diatoms and chocolate-brown algae, causing many destructive diseases to plants and animals (Beakes et al., 2012). Among the group, Phytophthora is the best-studied genus that includes over 100 species, which are divided into 10 clades (Kroon et al., 2012). Phytophthora parasitica Dastur (syn. P. nicotianae Breda de Haan) is classified in the Phytophthora clade 1 and its closest relatives include P. infestans (Blair et al., 2008; Kroon et al., 2012). Unlike the well-studied species P. infestans, which is a foliage pathogen and only infected few plants, P. parasitica is a typical root pathogen with a broad-range of host plants, being capable of infecting over 72 plant genera (Meng et al., 2014). The two species vary in genome sizes, beingness 82 and 240 Mb for P. parasitica and P. infestans, respectively (Judelson, 2012). However, well-nigh one-half of Phytophthora species are more often than not pathogenic on roots, and about 30% species being pathogens of multiple host plants (Kroon et al., 2012). Therefore, P. parasitica provides an opportunity for their office in agreement plant recognition and infection, and their wide host ranges. Moreover, while being a natural pathogen of tobacco species, P. parasitica is capable of infecting the model plant species Arabidopsis thaliana, which allows accelerated understanding of Phytophthora pathogenesis and plant susceptibility (Attard et al., 2010; Wang et al., 2011).
During co-development of institute host and the pathogen, plants take developed sophisticated recognition systems, especially the effector-triggered immunity (ETI; Jones and Dangl, 2006). The avirulence (Avr) genes from pathogen perceived direct or indirectly by the matching resistance (R) genes following the gene-for-cistron model, which leads to a rapid and enhanced defense response in the host found, frequently including hypersensitive response (Hour; Ali and Bakkeren, 2011). R gene-mediated recognition of pathogen effectors activates a serial of defense signaling cascades. So far, numerous R genes have been cloned from many constitute species (Liu et al., 2007). The largest class of known R proteins includes a nucleotide-bounden site and leucine-rich repeat domains (NBS-LRR proteins; Joshi and Nayak, 2013). A number of R genes have been cloned from the model species, A. thaliana, and have too been used extensively for answering cardinal questions in molecular plant–microbe interactions, including bacterial, viral, fungal, and oomycete pathogens (Nishimura and Dangl, 2010).
The established interactions between oomycetes and the model plant A. thaliana correspond an important contribution to the agreement of the oomycete pathogenicity mechanisms (Bozkurt et al., 2012). There take been several examples to use Arabidopsis to investigate found–oomycete pathogen interactions (Koch and Slusarenko, 1990; Holub et al., 1995; Roetschi et al., 2001; Robinson and Cahill, 2003; Daniel and Guest, 2006; Attard et al., 2010; Schlaeppi et al., 2010; Wang et al., 2011, 2013), peculiarly after the whole genome sequence of Arabidopsis was appear (Arabidopsis Genome Initiative [AGI], 2000). Among the pathosystems established, the best studied model, downy mildew, exhibits extensive variation with A. thaliana, which provides a rich resource for identification of at to the lowest degree 27 RPP genes (Coates and Beynon, 2010), from which several genes accept been cloned using map-based cloning. In dissimilarity, piffling is known about the Avr genes, with just four cloned, including ATR13 (Allen et al., 2004), ATR1 (Rehmany et al., 2005), ATR5 (Bailey et al., 2011), and ATR39-1 (Goritschnig et al., 2012). In addition, several functional resistance genes from potato and soybean, conferring resistance to P. infestans and P. sojae, respectively, take also been cloned. Most of the cloned R genes against oomycete pathogens belong to the NBS-LRR class of plant resistance genes (Gururani et al., 2012). More identified R genes and respective Avr genes have provided major insights into the mechanism of constitute–oomycete interactions (Stassen and Van den Ackerveken, 2011).
Still, these studies have been limited to race-specific resistance genes. The race-specific R genes are normally hard to provide long-lived resistance in the field, considering the encoded resistance is based on the recognition of corresponding Avr genes. For example, all of the 11 R genes originated from Solanum demissum accept lost resistance to P. infestans (Song et al., 2003). Broad-spectrum disease resistance, which refers to resistance against different pathogen species or the majority of races of one species, is desirable (Kou and Wang, 2010). Nevertheless, whether a broad-spectrum resistance gene is durable is still debatable. More than 100 illness-resistance genes take been cloned from different plant species (Liu et al., 2007), and in some cases, several R genes confer wide-spectrum resistance. For broad-spectrum affliction resistance, the beginning type is the resistance to ii or more unlike pathogens, and the second i is divers as the resistance to the majority races of the same pathogen. A expert example of the first type is the Arabidopsis R factor RPW8, which confers resistance to two different powdery mildew fungal pathogens, Erysiphe cruciferarum UEA1 and E. cichoracearum UCSC1 (Xiao et al., 2001). R genes of the second type have also been identified (Wang et al., 1996; Büschges et al., 1997). And besides, the cloned resistance gene RB from S. bulbocastanum enables potato highly resistant to all known races of P. infestans (Song et al., 2003), a subversive oomycete pathogen. WRR4 encodes a TIR-NB-LRR (Toll-similar/interleukin-ane receptor-nucleotide bounden-leucine-rich repeat) poly peptide, confers a dominant, wide-spectrum white rust resistance in Arabidopsis accretion Columbia to at least 4 races of Albugo candida, and requires expression of the lipase-like defense regulator, EDS1 (enhanced illness susceptibility ane; Borhan et al., 2008, 2010). However, the mechanisms of wide-spectrum affliction resistance are different. For example, the mlo resistance is a consequence of the recessive mutations in the barley Mlo locus (Büschges et al., 1997), while in some cases, the durability of R genes lie in the fitness toll in pathogen evolution to overcome the resistance (Vera Cruz et al., 2000). Bs2-mediated broad-spectrum disease resistance is achieved by recognition of avrBs2, an avirulence gene important in the fitness of Xanthomonas campestris pv. vesicatoria and highly conserved amongst other X. campestris pathovars (Tai et al., 1999). In the case of rice bacterial blight, the disease severity was loftier in 3 years on the Xa4 and Xa10 genotypes, simply not on the Xa7, as the mutation of avrXa7 was responsible for both the loss of avirulence function and reduced aggressiveness to rice (Vera Cruz et al., 2000). These results suggested that durability of R genes could be predicted according to the fitness or virulence contribution of respective avirulence genes.
In agronomical ecosystem, genetic resistance is the almost efficient form of protection against pathogens. R genes have been successfully used in crop improvement programs in the by and are being continuously exploited. Blackness shank, caused by P. parasitica, which is a subversive disease of tobacco worldwide, damages roots, stems, and leaves at whatsoever stages of tobacco growth. There are limited known sources of resistance against the pathogen. For instance, in the early on stage, the but available source of resistance was derived from Fla 301, which exhibited polygenic resistance. Dominant and monogenic resistance from Nicotiana plumbaginifolia Viv (Php) and N. longiflora Cav (Phl) was successfully incorporated into burley and flue-cured tobacco cultivars, respectively (Gutiérrez and Mila, 2007; Antonopoulos et al., 2010). However, cultivars with known resistance resources have failed to provide sufficient command confronting the pathogen in the field. Thus, there is a need to find new resistance resource. Autonomously from this, information technology is highly desirable to understand the found–pathogen interaction in order to develop novel disease-control strategies and improve disease-control measures.
Little is known almost the host specificities in P. parasitica although its interaction with tobacco plants follows a factor-for-cistron theory (Perrone et al., 2000) and with A. thaliana shows natural variation in host specificity (Wang et al., 2011). In this written report, we used detached foliage inoculation method to investigate phenotypic and genetic interaction betwixt A. thaliana and P. parasitica. Based on conscientious cess of variation in resistance phenotypes, we describe in this newspaper details of four interaction phenotypes. The phenotypic variations in A. thaliana to P. parasitica provide useful resources for meliorate understanding of the interaction between Phytophthora and the host plants. We too characterized the resistance of accession Zurich (Zu-1) to P. parasitica strain Pp016. Genetic analysis using segregating populations derived from a cross betwixt the resistant accession Zu-one and the hyper-susceptible accession Landsberg (Ler) show that the resistance is conditioned by a single semi-ascendant locus designated RPPA1 Zu-1 (for Resistance to P. parasitica 1). Since RPPA1 Zu-1 confers resistance to various P. parasitica strains, its hereafter cloning and assay volition facilitate improved understanding of broad-spectrum disease resistance in plants to oomycete pathogens.
Materials and Methods
Phytophthora parasitica Culture Conditions and Pathogenicity Assays
The P. parasitica strains were routinely cultured on 5% (v/v) cleared carrot juice agar (CA) medium supplemented with 0.002% (due west/five) β-sitosterol and 0.01% (due west/v) CaCO3 in the dark at 23°C. The P. parasitica zoospore grooming and pathogenicity assays were as described (Wang et al., 2011). For assessing the phenotype of Arabidopsis accessions to P. parasitica, at least ten plants were selected (ii leaves from each plant) for the pathogenicity assays. The determination of a whole accretion every bit a specific phenotype is based on the infection of leaves of majority plants (more than seventy–80%). All experiments were repeated three times.
Arabidopsis thaliana Growing Weather
The preservation of seeds followed a standard protocol (Martínez-Zapater and Salinas, 1998). For the discrete foliage inoculation experiments, the plants were grown in soil at xx–25°C with a photoperiod of 12 h 24-hour interval/nighttime and discrete leaves of 28–xxx days-quondam seedlings were used for inoculation. For whole bulb inoculation experiments, the plants were sown on half-force MS plates and were grown in a bedchamber at 22°C with a photoperiod of 12 h day/night and two weeks-old plants were used for inoculation.
Microscopic Examination
To visualize the P. parasitica infection structures and plant cell death, foliage, and root tissues were harvested at different fourth dimension points and stained using a modified trypan blue method as described by Wang et al. (2011). The samples were mounted in 50% glycerol and viewed under Olympus BX-51 microscope equipped with differential interference contrast (DIC) eyes (Olympus, Japan). For microscopic label of infection with P. parasitica transformant 1121, which stably expresses green fluorescent protein (GFP), infected tissues were nerveless and viewed under Olympus BX-51 fluorescent microscope with the GFP filter (BP450–BP480).
Callose Deposition Staining
To visualize callose deposition, the A. thaliana leaf tissues were stained with aniline blueish (Bouwmeester et al., 2011). After inoculation, the tissues were cleared overnight in 96% ethanol and stained with i% (w/5) aniline bluish in 150 mM Grand2HPOfour (pH 9.5) for 1 h. The samples were mounted on glass microscope slides in 50% glycerol and viewed nether Olympus BX-51 fluorescent microscope with the UV filter (BP330–BP385).
Association Mapping of RPPA1Zu-ane Using SLAF-seq
Two phenotypically contrasting bulks each comprising 30 Fii plants, one resistant and the other susceptible to P. parasitica were generated. Including the parent pools, we totally sequenced four pools for determination of the regions associated with resistance phenotype. The raw sequencing data accept been deposited to the Sequenced Read Archive (Bioproject number: PRJNA282643). The accession numbers for the parent accessions Zu-1 and Ler-i, and the resistant and susceptible bulks are SRR2002811, SRR2002808, SRR2002809, and SRR2002285, respectively. The specific-locus amplified fragment sequencing (SLAF-seq) procedure (Biomarker, Beijing, China) was performed as described (Lord's day et al., 2013). For genome-broad association analysis, all the markers were parsed as multiple contingency tables and Fisher's exact tests were used to calculate the associations of the makers with the resistance locus. Multiple tests were controlled by false discovery rate using a Bonferroni procedure as developed in the R plan (http://www.r-project.org).
Results
Phenotypic Variations in Arabidopsis thaliana to Phytophthora parasitica Pp016
In a previous study (Wang et al., 2011), nosotros examined the interaction phenotypes of A. thaliana accessions to P. parasitica strain Pp016. By using root inoculation method, well-nigh all of the 25 A. thaliana accessions were susceptible. Yet, by infection of detached leaves, some of the 20 briefly examined accessions exhibited varying degrees of resistance. To further investigate the resistance on the leaves of A. thaliana response to P. parasitica, we examined additional 28 accessions by inoculation of detached leaves with P. parasitica Pp016, and examined the extent of disease evolution macroscopically and microscopically 3 days post inoculation (dpi). The results showed that the 48 (including 20 accessions briefly examined in previous study) accessions (Table 1) tested can be categorized into iv distinct groups (N, Y, W, H) according to the resistance response, affliction severity scored with water-soaked lesion size, development of arable haustoria, and production of sporangia. The interaction phenotypes were characterized as follows:

Table 1. Phenotypic variation of Arabidopsis thaliana accessions (48) to Phytophthora parasitica strain Pp016.
Phenotype N (Figure 1A): salubrious leaves with no visible affliction symptoms and most no pathogen colonized at the inoculation site (Figure 1B), but Northwardecrotic flecks on the leaf surface observed under a dissecting microscope at low magnification (Figure 1I). Phenotype Y (Figure 1C): Yellowing surrounds inoculation sites within one-third of foliage sizes with spare hyphae (Effigy 1D) and occasional visible haustoria observed. Phenotype W (Figure 1E): Water-soaked lesions on leaves with heavy hyphae colonized (Figure 1F) and numerous haustoria formed 3 dpi, and past 4 dpi abundant sporangia formed on the leafage surface equally described (Wang et al., 2011). Phenotype H (Effigy 1G): Hyper-susceptible phenotype exhibited the whole leaves watered with heavier hyphae colonized (Figure 1H) and extremely abundant haustoria (Effigy 1J) formed 3 dpi, and also the sporangia formed earlier than phenotype W on the leaf surface.
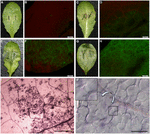
Figure ane. Variation of interaction phenotypes of Arabidopsis thaliana accessions inoculated with Phytophthora parasitica strain Pp016. Leaves of 4 weeks-old A. thaliana seedlings were inoculated with zoospores of P. parasitica strain Pp016 (1 × 10v zoospores/mL) and the phenotype scored iii days post inoculation (dpi). To access the extent of pathogen infection, P. parasitica transformant 1121 that stably expresses green fluorescent poly peptide (GFP) was used for inoculation. (A) Phenotype Northward, no visible symptom on the leaf surface (Zu-1). (C) Phenotype Y, yellowish around the restricted water-soaked lesions that are smaller than 1-third leaf sizes (Et-0). (E) Phenotype W, water-soaked lesions (Col-0). (G) Phenotype H, hyper-susceptible with the whole leaves water-soaked (Ler). (B,D,F,H) Cytological characterization of hyphae colonization in (A,C,Eastward,G), respectively, by P. parasitica strain 1121 (bar, 500 μm). (I) Necrotic flecks were observed under a dissecting microscope at low magnification (iv×) (bar, 500 μm). (J) Extremely abundant haustoria formed in mesophyll cells in the phenotype H (bar, 50 μm).
Accession Zu-1 exhibited the well-nigh resistance to Pp016, and was classified as the phenotype N. The leaves remained light-green, similar to the control leaves for up to 6 dpi. Ix of 48 accessions were moderately resistant, being classified every bit the phenotype Y, with restricted water-soaked lesions only smaller than one-third of leafage sizes by iii dpi. Near A. thaliana accessions (35 of 48) were susceptible to Pp016 (scored in this study as the phenotype West), similar to the accession Col-0 as described (Wang et al., 2011). Three accessions, including Ler, were the most susceptible to Pp016 (phenotype H), with whole leaf water-soaked and heavily colonized by 3 dpi.
Zu-1 is Highly Resistant to a Gear up of Diverse P. parasitica Strains
Of the 48 accessions tested, Zu-ane exhibited the almost resistant phenotype to Pp016. To investigate whether the Zu-one resistance phenotype is wide-spectrum, we performed infection assays with additional 19 P. parasitica strains isolated from different host plants (Table two). The results indicated that Zu-1 is highly resistant to all of examined strains. Accretion Ler is susceptible to all the strains except Pp008 and Pp009, and hyper-susceptible to a half of strains tested. Accretion Col-0 exhibited dissimilar phenotypes to the 20 examined P. parasitica strains.
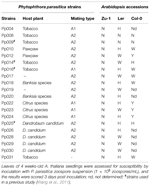
Tabular array ii. Phenotypic characterization of A. thaliana accessions Zu-1, Ler, and Col-0 to 20 P. parasitica strains.
Microscopic Characterization of Resistance in Zu-1 to P. parasitica
To further understand the resistance of Zu-1 to P. parasitica, we inoculated Zu-1 and the susceptible accretion Ler with zoospores of P. parasitica strain Pp016 and examined for cellular reactions at different fourth dimension points microscopically, later on the infected tissues were stained with trypan blue. As shown in Figure 2, at the initial 3 hours post inoculation (hpi), there were no differences between Zu-1 and Ler in response to P. parasitica infection, including the timing of cyst formation, the rates of cyst germination and development of appressoria (Figures 2A–C). Even so, starting from when the penetration pegs emerged beneath the appressoria and the penetration hyphae started to grow between the anticlinal walls of 2 epidermal cells, differences between Zu-1 and Ler became apparent. In the susceptible Ler plants, the penetration procedure was similar to Col-0 equally described (Wang et al., 2011). Withal, many more haustoria formed and abundant sporangia produced earlier in Ler than that in Col-0, which is indicative of heavy colonization by strain Pp016. In resistant Zu-1 plants, the earliest microscopically visible response was observed at six hpi, when cysts germinated and developed appressoria. At the attempted penetration sites of Zu-1 by Pp016, papilla appeared with heavy deposition of callose materials (Figures 2D–F), equally revealed by aniline bluish staining. Generally, the wall of some epidermal cells was encased with callose (Figures 2G,H). HR is the most important phenotype in the resistant plants against pathogens. Typically, only a few epidermal cells that were penetrated by Pp016 showed a cell death response. This was frequently followed by, at nearly all the infection sites, rapid HRs, which limited in single or a few epidermal cells (Figure 2I), or stoma baby-sit cells (Effigy 2J), or stoma guard cells and an next epidermal cells (Figure 2K). This necrotic response typically stops P. parasitica infection (Figure 2L).
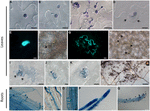
FIGURE 2. Cytological label of infection of A. thaliana accession Zu-1 to P. parasitica Pp016. (A) Zoospores encysted at 30 min (bar, 10 μm). (B) Cyst germinated with a germ tube at ane.5 hpi (bar, 10 μm). (C) Cyst germinated and appressorium formed at 3 hpi (bar, 20 μm). (D) Heavy deposition of materials surrounding the attempted penetration sites (arrows) at the edge of 2 anticlinal walls of epidermal cells (bar, 10 μm). (E) Fluorescence of callose at the penetration sites along the junction between epidermal jail cell walls (bar, 10 μm). (F) Bright-field paradigm of (East), showing a germinated cyst at the penetration site (bar, ten μm). Arrows point to the place where the cyst is visible. (Thousand) Fluorescence and thickening of the prison cell wall showing an infected epidermal cell (bar, 20 μm). (H) Bright-field paradigm of (Chiliad) showing the germinated cysts at the penetration sites (bar, twenty μm). Arrows point to places where the cysts are visible. (I–Thousand) Hypersensitive response (HR) of an epidermal cell (bar, 20 μm), stoma guard cells (bar, 10 μm), and an epidermal cell shut to the stoma (bar, 20 μm), in response to penetration past Pp016 at 48 hpi. Arrows signal the heavy deposit of cloth surrounding the attempted penetration site in epidermal cells. (Fifty) Necrosis on the leaf surface 72 hpi and the hypersensitive cells are stained as darker color (bar, 200 μm). (M) Zoospores germinated and formed appressoria at half-dozen hpi (bar, x μm). (N,O) Haustoria-similar structures adult in the cortex at 12 hpi (bar, 10 μm). (P) Heavy hyphal colonization in roots at 48 hpi (bar, 100 μm). (Q) Sporangia produced on the root surface at 72 hpi (bar, 100 μm).
Zu-1 is Susceptible to Root Infection past P. parasitica
Since P. parasitica is a typical root pathogen of many plants, nosotros also tested whether Zu-i is resistant to root infection. Live root tissues were inoculated by dipping into a zoospore pause. The consequence showed that Zu-1 is susceptible to P. parasitica, with the inoculated seedlings wilted and collapsed 4 dpi. Compared with Ler and Col-0, Zu-1 roots exhibited no clear differences to P. parasitica infection. The microscopic characterization showed that the infection process past P. parasitica on Zu-1 was similar to that on Ler and Col-0. Zoospores geminated 1.v hpi and most all zoospores developed germ tubes (Figure 2M) at 6 hpi. Colonization by invasive hyphae was observed both within the cells and in the intercellular spaces of the root tissues, and haustoria-like structures (Figure 2N) were formed in the cortex at 12 hpi. And in many cases, the haustoria-like structures in roots were as wide as the hyphae they originated from (Effigy 2O). By 48 hpi, heavy P. parasitica hyphae colonization in root tissues was apparent (Figure 2P) and, by 72 hpi, numerous sporangia were visible (Effigy 2Q).
Genetic Analysis of Resistance in Zu-ane to P. parasitica
To examine inheritance of resistance in Zu-one, genetic crosses were carried out using Zu-1 and the fully susceptible Ler. The obtained 25 F1 plants and the F2 populations (Table 3) were inoculated with Pp016 and scored for response phenotypes 3 dpi. The results showed that 25 Fone progenies exhibited a moderate resistant response (Phenotype Y) with some phenotypic variations to Pp016. Compared with the parents Zu-1 (Effigy 1A) and Ler (Figure 1C), the water-soaked lesions of F1 progenies were restricted, mostly inside (Effigy 3A) or a little more than one-third of leaf sizes (Figure 3B). The surrounding leaf tissues became xanthous 3 dpi, just the colonizing hyphae were restricted inside the inoculation sites. The extent of P. parasitica colonization in Fane progenies with moderately resistant response was too examined microscopically. The invasive hyphae observed were sparse (Figure 3C). Compared with abundant haustoria formed in Ler (Figure 1J), fewer haustoria were formed in the mesophyll cells from the intercellular penetrating hyphae in Fane progenies (Figure 3D). Numerous cell expiry in the epidermal cells also occurred (Figure 3E) but not as many every bit observed for the parent Zu-one, in which nearly all the penetrated epidermal cells responded with cell death.

TABLE 3. Genetic analysis of resistance in A. thaliana Zu-1 to P. parasitica Pp016.
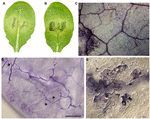
FIGURE 3. Phenotypic characterizations of F1 progenies of Zu-1 and Ler to infection by P. parasitica strain Pp016. Leaves of 4 weeks-old A. thaliana seedlings were inoculated with zoospores of P. parasitica strain Pp016 (ane × 105 zoospores/mL) and the phenotype scored three dpi. (A,B) Leaves of F1 progenies are moderately resistant with restricted water-soaked lesions. (C) Less colonized hyphae in epidermal cells (bar, 200 μm). (D) Less colonized hyphae in mesophyll cells with few haustoria (bar, 50 μm). Arrows indicate haustoria-similar structures. (Eastward) Hour in epidermal cells (bar, twenty μm).
Segregation analysis of F2 populations showed that one quarter (47/176) were susceptible to Pp016, like to Ler, while some other quarter (42/176) were similar to Zu-1 being resistant to Pp016 (Table three). Virtually one-half (87/176) of F2 populations showed the F1 phenotype, being moderately resistant. The segregation (North: Y: Southward∗) ratio was very shut to 1: 2: 1 (χ2 = 0.307, P = 0.8578) in F2 populations, consistent with the interpretation that a unmarried semi-dominant locus confers resistance in Zu-1 to P. parasitica Pp016. The locus is designated every bit RPPA1 Zu-one (Resistance to P. parasitica 1). Also, inoculation of the F2 populations with another P. parasitica strain Pp025 revealed 100% correlation of phenotypes that was observed with strain Pp016. This indicates that the RPPA1 Zu-1 locus confers resistance to at least ii strains of P. parasitica.
Preliminary Mapping of RPPA1 Zu-i Using SLAF-seq
By employing SLAF-seq method (Sun et al., 2013), we sequenced four samples, including ii parents and two F2 bulked populations. As the Ftwo populations were obtained by selfing the F1 progeny of a cross between two fully homozygous parents with the genotypes RR or rr, a total of 2742 polymorphic SLAFs were detected and were analyzed for the clan with resistance in accession Zu-1 to P. parasitica strain Pp016. The results farther showed that the strongest associated region was located between 7.ane and eleven.two Mb in the chromosome Iv (Figure 4).

Figure iv. Clan mapping analysis of RPPA1 Zu-1 by SLAF-seq. The adjusted P values for the markers associated with the resistance locus were showed in a logarithmic scale.
Discussion
Elucidation of the mechanism of the interaction between the pathogen and host plants has been the focus for understanding of illness resistance. The objective of this research is to investigate phenotypic and genetic variations in A. thaliana to P. parasitica. In this paper, we inoculated 28 A. thaliana accessions with P. parasitica strain Pp016 and variation in resistance phenotypes was evident. Based on careful assessment of variation in resistance phenotypes to P. parasitica strain Pp016, the 48 accessions tin can be divided into four distinct groups. We establish that near accessions (38/48) are susceptible to Pp016, including three hyper-susceptible accessions. 9 of 48 accessions are resistant. Nosotros as well showed that Zu-1 is highly resistant to all twenty strains tested. The genetic analysis of segregating populations derived from cross Zu-ane and Ler showed that the resistance to at least two of P. parasitica strains is conditioned by a single semi-ascendant locus.
A. thaliana accretion Zu-1 is resistant to xx P. parasitica strains isolated from unlike host plants, including tobacco, pawpaw, Banksia species, Citrus species, and Dendrobium candidum. Apart from the origin the P. parasitica strains were isolated, the strain option was also based on their dandy differences in virulence spectrum on a set of 12 tobacco cultivars (data non shown). For example, strain Pp014 is virulent to all 12 tobacco cultivars, Pp009 is virulent to only three of them, and Pp016 is virulent to eight of them.
Zu-ane was highly resistant to all examined P. parasitica strains and microscopically exhibited strong cell death response. Resistance in Zu-one was manifested as necrosis of found cells in the epidermal layer (Figures 2I–K), which comprised only one or a few epidermal cells, and the pathogen was not observed in the mesophyll cells, which is different from the resistance phenotype of Col-0 in response to infection with P. parasitica strain Pp009 (Wang et al., 2011), and other Phytophthora pathogens on A. thaliana (Roetschi et al., 2001; Wang et al., 2013), in which the mesophyll cells undergoing Hour to finish the penetration of progressive hyphae. The diverse range of reaction phenotypes of unlike plant–pathogen combinations also occurs in A. thaliana to the oomycete pathogen Hyaloperonospora arabidopsidis (Koch and Slusarenko, 1990; Parker et al., 1993; Reignault et al., 1996). The different responses of known resistance were due to the presence of dissimilar resistance loci. The thickening of the cell wall and the germination of callose-containing papillae were axiomatic in Zu-1, which were mutual characteristics in other plant resistance responses (Figures Figure second–H). Massive callose depositions were observed at the penetration sites and in the cells undergoing HR (Effigy 2I). These results advise that resistance in Zu-1 is triggered at early stage of infection. In addition, of the described A. thaliana and Phytophthora interactions (Wang et al., 2011, 2013), the failure of the start pathogen penetration often leads to the development of secondary germ tubes and appressoria. Interestingly, we did not observe this miracle in Zu-1. Furthermore, at the initial 6 hpi, cyst germination and appressorial evolution were like to that on the susceptible Ler.
Zu-i is susceptible to root infection past P. parasitica strain Pp016, which is very different from its highly resistant to leaf infection. Actually, with root inoculation method, about accessions of A. thaliana cannot prevent the P. parasitica pathogen (Attard et al., 2010; Wang et al., 2011). It is axiomatic that unlike plant organs may elicit the activation of specific signaling networks. Organ-specificity of defence responses in plant disease has been described (Hermanns et al., 2003; Rookes et al., 2008). A particular pathogen only infects some organs of a genetically susceptible host only not other organs or the unabridged constitute. Every bit described in many establish–pathogen interactions, the found defense hormones salicylic acid (SA), jasmonic acid (JA), and ethylene (ET) accept been shown to play important roles in the resistance reaction. SA-signaling is important for defence force against biotrophic pathogens, while JA and/ or ET-signaling is involved in defense against necrotrophic pathogens, although there are exceptions and additional complexities (Glazebrook, 2005). During root infection by P. parasitica, SA, JA, and ET signaling pathways cooperate in the defenses (Attard et al., 2010), which is similar with Arabidopsis defense response against the root fungal pathogen Fusarium oxysporum (Berrocal-Lobo and Molina, 2008), but is different from other Phytophthora pathogens on Arabidopsis (Roetschi et al., 2001; Rookes et al., 2008; Schlaeppi et al., 2010; Wang et al., 2013). Notwithstanding, most of the results were obtained from the leaf infections. The underlying mechanisms mediating differences of defense pathways in host plants and the organ-specificity are complicated. Zu-i exhibited obvious differences in resistance to P. parasitica and may provide an excellent model for understanding organ-specific affliction resistance.
Since semi-dominance is a widespread characteristic of resistance genes and the mechanistic implications have been discussed (Crute and Norwood, 1986). For example, the get-go resistance factor RPP5 from A. thaliana to H. arabidopsidis was identified equally a semi-ascendant factor, which resulted in resistance in the heterozygote being lower than in the resistant homozygote (Parker et al., 1993, 1997). In our observation, the moderate resistant phenotype of F1 plants of Zu-one and Ler exhibits yellowish characters macroscopically and restricted lesion development. In addition, the Ftwo populations were observed with a ratio very close to i: 2: i (χ2 = 0.307, P = 0.8578). Based on the phenotype of F1 progenies and the Ftwo segregation data, the resistance in Zu-1 to P. parasitica Pp016 seemed to be conditioned by a semi-dominant locus, RPPA1 Zu-1 . Still, the heterozygous RPPA1 Zu-ane in F2 plants to P. parasitica exhibited intermediate phenotype, making it difficult to achieve fine concrete mapping of the RPPA1 Zu-one locus. Analysis of selected F3 progenies would be necessary to assign accurately a genotype of the plants.
Recent developments in loftier throughput next-generation sequencing technologies now can provide new strategies for sequence-based genotyping. Several methods have been adult that involve sequencing simply a small fraction of the entire genome. Specific-locus amplified fragment sequencing (SLAF-seq) approach is a strategy developed for the de novo SNP discovery and genotyping of large populations using an enhanced RRL sequencing method (Sun et al., 2013). And this method has been used for haplotype mapping, genetic mapping, linkage mapping, and polymorphism mapping (Chen et al., 2013; Sun et al., 2013; Zhang et al., 2013; Chen et al., 2014). In this study, we employed the SLAF-seq approach to map the locus RPPA1 Zu-1 . Clan analysis preliminarily determined the RPPA1 Zu-1 locus in a region between 7.1 and 11.2 Mb in the chromosome IV.
Phenotypic variation was characterized among the interaction with respect to the extent of pathogen colonization and the host response. The timing and degree of pathogen infection and colonization vary among the combinations. HRs range from occasionally in some pocket-size regions to almost each infection epidermal cells. The observed phenotypic variations provide a useful resources for investigating molecular process in the interaction between A. thaliana and P. parasitica. The primary identification and hereafter cloning of the RPPA1 Zu-one locus may offer a valuable model for understanding the broad-spectrum resistance in plants against oomycete pathogens.
Author Contributions
Conceived and designed the experiments: WS; Performed the experiments: YM YH, QZ, and GH; Analyzed the data: WS, YM, QW, and JJ; Contributed reagents/materials/analysis tools: YM, YH, QZ, GH, JQ, and QW; Wrote the paper: YM and WS, with contribution from all authors.
Conflict of Interest Statement
The authors declare that the inquiry was conducted in the absence of any commercial or fiscal relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Dr. Cai-Zhong Jiang of University of California at Davis for providing the A. thaliana stocks, Professor Adrienne Hardham of Australian National University and Dr. Jingze Zhang of Zhejiang University for P. parasitica strains. We also thank Dr. Wei Liu for useful suggestions and discussions. This piece of work was supported by National Natural Scientific discipline Foundation of China (#31125022) and People's republic of china Agriculture Research System (CARS-x).
References
Ali, Southward., and Bakkeren, G. (2011). Fungal and oomycete effectors-strategies to subdue a host. Tin can. J. Plant Pathol. 33, 425–446. doi: 10.1080/07060661.2011.625448
CrossRef Full Text | Google Scholar
Allen, R. L., Bittner-Boil, P. D., Grenville-Briggs, 50. J., Meitz, J. C., Rehmany, A. P., Rose, L. Eastward., et al. (2004). Host-parasite coevolutionary conflict between Arabidopsis and downy mildew. Science 306, 1957–1960. doi: 10.1126/science.1104022
PubMed Abstract | CrossRef Full Text | Google Scholar
Antonopoulos, D. F., Melton, T., and Mila, A. L. (2010). Effects of chemical control, cultivar resistance, and structure of cultivar root system on black shank incidence of tobacco. Plant Dis. 94, 613–620. doi: 10.1094/PDIS-94-five-0613
CrossRef Total Text | Google Scholar
Attard, A., Gourgues, M., Callemeyn-Torre, N., and Keller, H. (2010). The firsthand activation of defence responses in Arabidopsis roots is not sufficient to prevent Phytophthora parasitica infection. New Phytol. 187, 449–460. doi: 10.1111/j.1469-8137.2010.03272.x.
PubMed Abstract | CrossRef Full Text | Google Scholar
Bailey, K., Çevik, Five., Holton, N., Byrne-Richardson, J., Sohn, 1000. H., Coates, M., et al. (2011). Molecular cloning of ATR5Emoy2 from Hyaloperonospora arabidopsidis, an avirulence determinant that triggers RPP5-mediated defense in Arabidopsis. Mol. Institute Microbe Interact. 24, 827–838. doi: 10.1094/MPMI-12-10-0278
PubMed Abstract | CrossRef Full Text | Google Scholar
Blair, J. Eastward., Coffey, Yard. D., Park, S.-Y., Geiser, D. 1000., and Kang, South. (2008). A multi-locus phylogeny for Phytophthora utilizing markers derived from consummate genome sequences. Fungal Genet. Biol. 45, 266–277. doi: 10.1016/j.fgb.2007.10.010
PubMed Abstruse | CrossRef Full Text | Google Scholar
Borhan, M. H., Gunn, N., Cooper, A., Gulden, S., Tör, Yard., Rimmer, S. R., et al. (2008). WRR4 encodes a TIR-NB-LRR protein that confers broad-spectrum white rust resistance in Arabidopsis thaliana to iv physiological races of Albugo candida. Mol. Plant Microbe Interact. 21, 757–768. doi: 10.1094/MPMI-21-6-0757
PubMed Abstruse | CrossRef Full Text | Google Scholar
Borhan, Grand. H., Holub, East. B., Kindrachuk, C., Omidi, K., Bozorgmanesh-Frad, Chiliad., and Rimmer, S. R. (2010). WRR4, a broad-spectrum TIR-NB-LRR gene from Arabidopsis thaliana that confers white rust resistance in transgenic oilseed brassica crops. Mol. Plant Pathol. 11, 283–291. doi: x.1111/j.1364-3703.2009.00599.x
PubMed Abstract | CrossRef Full Text | Google Scholar
Bouwmeester, G., de Sain, M., Weide, R., Gouget, A., Klamer, S., Canut, H., et al. (2011). The lectin receptor kinase LecRK-I.9 is a novel Phytophthora resistance component and a potential host target for a RXLR effector. PLoS Pathog. vii:e1001327. doi: 10.1371/journal.ppat.1001327
PubMed Abstract | CrossRef Full Text | Google Scholar
Bozkurt, T. O., Schornack, S., Banfield, M. J., and Kamoun, S. (2012). Oomycetes, effectors, and all that jazz. Curr. Opin. Plant Biol. xv, 483–492. doi: 10.1016/j.pbi.2012.03.008
PubMed Abstract | CrossRef Full Text | Google Scholar
Büschges, R., Hollricher, Thousand., Panstruga, R., Simons, G., Wolter, M., Frijters, A., et al. (1997). The barley Mlo gene: a novel command element of plant pathogen resistance. Cell 88, 695–705. doi: 10.1016/S0092-8674(00)81912-i
PubMed Abstract | CrossRef Full Text | Google Scholar
Chen, South., Huang, Z., Dai, Y., Qin, S., Gao, Y., Zhang, Fifty., et al. (2013). The development of 7E chromosome-specific molecular markers for Thinopyrum elongatum based on SLAF-seq technology. PLoS I viii:e65122. doi: 10.1371/periodical.pone.0065122
PubMed Abstract | CrossRef Total Text | Google Scholar
Chen, Due west., Yao, J., Chu, L., Li, Y., Guo, X., and Zhang, Y. (2014). The development of specific SNP markers for chromosome 14 in cotton using adjacent-generation sequencing. Plant Breed. 133, 256–261. doi: 10.1111/pbr.12144
CrossRef Full Text | Google Scholar
Crute, I. R., and Norwood, J. Yard. (1986). Gene-dosage effects on the relationship betwixt Bremia lactucae (downy mildew) and Lactuca sativa (lettuce): the relevance to a mechanistic understanding of host-parasite specificity. Physiol. Mol. Plant Pathol. 29, 133–145. doi: x.1016/S0048-4059(86)80016-9
CrossRef Total Text | Google Scholar
Daniel, R., and Guest, D. (2006). Defence responses induced by potassium phosphonate in Phytophthora palmivora-challenged Arabidopsis thaliana. Physiol. Mol. Plant Pathol. 67, 194–201. doi: 10.1016/j.pmpp.2006.01.003
CrossRef Full Text | Google Scholar
Glazebrook, J. (2005). Contrasting mechanisms of defense confronting biotrophic and necrotrophic pathogens. Annu. Rev. Phytopathol. 43, 205–227. doi: ten.1146/annurev.phyto.43.040204.135923
PubMed Abstruse | CrossRef Full Text | Google Scholar
Goritschnig, S., Krasileva, K. 5., Dahlbeck, D., and Staskawicz, B. J. (2012). Computational prediction and molecular label of an oomycete effector and the cognate Arabidopsis resistance gene. PLoS Genet. viii:e1002502. doi: 10.1371/journal.pgen.1002502
PubMed Abstract | CrossRef Full Text | Google Scholar
Gururani, M. A., Venkatesh, J., Upadhyaya, C. P., Nookaraju, A., Pandey, S. K., and Park, S. W. (2012). Constitute disease resistance genes: electric current status and futurity directions. Physiol. Mol. Constitute Pathol. 78, 51–65. doi: 10.1016/j.pmpp.2012.01.002
CrossRef Total Text | Google Scholar
Gutiérrez, W. A., and Mila, A. Fifty. (2007). A rapid technique for determination of races of Phytophthora nicotianae on tobacco. Institute Dis. 91, 985–989. doi: x.1094/PDIS-91-8-0985
CrossRef Total Text | Google Scholar
Hermanns, G., Slusarenko, A. J., and Schlaich, N. L. (2003). Organ-specificity in a plant disease is determined independently of R factor signaling. Mol. Plant Microbe Collaborate. 16, 752–759. doi: ten.1094/MPMI.2003.16.nine.752
PubMed Abstract | CrossRef Total Text | Google Scholar
Holub, E. B., Brose, Eastward., Tör, G., Dirt, C., Crute, I. R., and Beynon, J. L. (1995). Phenotypic and genotypic variation in the interaction between Arabidopsis thaliana and Albugo candida. Mol. Plant Microbe Interact. 8, 916–928. doi: 10.1094/MPMI-viii-0916
PubMed Abstract | CrossRef Full Text | Google Scholar
Joshi, R. One thousand., and Nayak, S. (2013). Perspectives of genomic diversification and molecular recombination towards R-gene development in plants. Physiol. Mol. Biol. Plants nineteen, 1–9. doi: 10.1007/s12298-012-0138-ii
PubMed Abstract | CrossRef Total Text | Google Scholar
Liu, J., Liu, X., Dai, Fifty., and Wang, G. (2007). Recent progress in elucidating the structure, function and evolution of disease resistance genes in plants. J. Genet. Genomics 34, 765–776. doi: 10.1016/S1673-8527(07)60087-3
PubMed Abstruse | CrossRef Full Text | Google Scholar
Parker, J. E., Coleman, Thou. J., Szabò, V., Frost, L. N., Schmidt, R., Van der Biezen, E. A., et al. (1997). The Arabidopsis downy mildew resistance gene RPP5 shares similarity to the cost and interleukin-i receptors with North and L6. Constitute Cell 9, 879–894. doi: x.1105/tpc.9.6.879
PubMed Abstruse | CrossRef Total Text | Google Scholar
Parker, J. E., Szabò, V., Staskawicz, B. J., Lister, C., Dean, C., Daniels, Chiliad. J., et al. (1993). Phenotypic label and molecular mapping of the Arabidopsis thaliana locus RPP5, determining disease resistance to Peronospora parasitica. Plant J. iv, 821–831. doi: 10.1046/j.1365-313X.1993.04050821.x
CrossRef Full Text | Google Scholar
Perrone, S., Bui, F., Sutherland, M., and Guest, D. (2000). Gene-for-cistron specificity expressed in planta is preserved in cell cultures of Nicotiana tabacum inoculated with zoospores of Phytophthora nicotianae. Physiol. Mol. Found Pathol. 57, 235–242. doi: 10.1006/pmpp.2000.0300
CrossRef Full Text | Google Scholar
Rehmany, A. P., Gordon, A., Rose, L. E., Allen, R. L., Armstrong, M. R., Whisson, Due south. C., et al. (2005). Differential recognition of highly divergent downy mildew avirulence factor alleles by RPP1 resistance genes from two Arabidopsis lines. Plant Cell 17, 1839–1850. doi: ten.1105/tpc.105.031807
PubMed Abstract | CrossRef Total Text | Google Scholar
Reignault, P., Frost, L. N., Richardson, H., Daniels, K. J., Jones, J. D., and Parker, J. East. (1996). Four Arabidopsis RPP loci decision-making resistance to the Noco2 isolate of Peronospora parasitica map to regions known to contain other RPP recognition specificities. Mol. Institute Microbe Collaborate. 9, 464–473. doi: 10.1094/MPMI-9-0464
PubMed Abstruse | CrossRef Full Text | Google Scholar
Robinson, L. H., and Cahill, D. M. (2003). Ecotypic variation in the response of Arabidopsis thaliana to Phytophthora cinnamomi. Australas. Plant Pathol. 32, 53–64. doi: x.1071/AP02064
CrossRef Full Text | Google Scholar
Roetschi, A., Si-Ammour, A., Belbahri, L., Mauch, F., and Mauch-Mani, B. (2001). Characterization of an Arabidopsis-Phytophthora pathosystem: resistance requires a functional PAD2 factor and is contained of salicylic acid, ethylene and jasmonic acid signalling. Institute J. 28, 293–305. doi: 10.1046/j.1365-313X.2001.01148.x
PubMed Abstruse | CrossRef Total Text | Google Scholar
Rookes, J. E., Wright, Thou. L., and Cahill, D. M. (2008). Elucidation of defence force responses and signalling pathways induced in Arabidopsis thaliana following challenge with Phytophthora cinnamomi. Physiol. Mol. Plant Pathol. 72, 151–161. doi: ten.1016/j.pmpp.2008.08.005
CrossRef Full Text | Google Scholar
Schlaeppi, K., Abou-Mansour, E., Buchala, A., and Mauch, F. (2010). Disease resistance of Arabidopsis to Phytophthora brassicae is established by the sequential action of indole glucosinolates and camalexin. Plant J. 62, 840–851. doi: ten.1111/j.1365-313X.2010.04197.x
PubMed Abstract | CrossRef Total Text | Google Scholar
Song, J., Bradeen, J. Yard., Naess, Southward. K., Raasch, J. A., Wielgus, S. M., Haberlach, K. T., et al. (2003). Gene RB cloned from Solanum bulbocastanum confers broad spectrum resistance to potato late bane. Proc. Natl. Acad. Sci. U.S.A. 100, 9128–9133. doi: 10.1073/pnas.1533501100
PubMed Abstract | CrossRef Full Text | Google Scholar
Dominicus, X., Liu, D., Zhang, X., Li, W., Liu, H., Hong, W., et al. (2013). SLAF-seq: an efficient method of large-scale De novo SNP discovery and genotyping using high-throughput sequencing. PLoS Ane viii:e58700. doi: 10.1371/journal.pone.0058700
PubMed Abstruse | CrossRef Total Text | Google Scholar
Tai, T. H., Dahlbeck, D., Clark, E. T., Gajiwala, P., Pasion, R., Whalen, Grand. C., et al. (1999). Expression of the Bs2 pepper cistron confers resistance to bacterial spot disease in tomato plant. Proc. Natl. Acad. Sci. U.S.A. 96, 14153–14158. doi: 10.1073/pnas.96.24.14153
PubMed Abstract | CrossRef Total Text | Google Scholar
Vera Cruz, C. M., Bai, J., Oña, I., Leung, H., Nelson, R. J., Mew, T. W., et al. (2000). Predicting durability of a illness resistance cistron based on an cess of the fitness loss and epidemiological consequences of avirulence gene mutation. Proc. Natl. Acad. Sci. U.S.A. 97, 13500–13505. doi: x.1073/pnas.250271997
PubMed Abstract | CrossRef Full Text | Google Scholar
Wang, One thousand. L., Song, Due west. Y., Ruan, D. Fifty., Sideris, S., and Ronald, P. C. (1996). The cloned gene, Xa21, confers resistance to multiple Xanthomonas oryzae pv oryzae isolates in transgenic plants. Mol. Plant Microbe Interact. 9, 850–855. doi: ten.1094/MPMI-nine-0850
PubMed Abstract | CrossRef Full Text | Google Scholar
Wang, Y., Bouwmeester, K., Van de Mortel, J. Due east., Shan, W., and Govers, F. (2013). A novel Arabidopsis-oomycete pathosystem: differential interactions with Phytophthora capsici reveal a role for camalexin, indole glucosinolates and salicylic acid in defence force. Plant Cell Environ. 36, 1192–1203. doi: 10.1111/pce.12052
PubMed Abstract | CrossRef Full Text | Google Scholar
Wang, Y., Meng, Y., Zhang, M., Tong, Ten., Wang, Q., Sun, Y., et al. (2011). Infection of Arabidopsis thaliana by Phytophthora parasitica and identification of variation in host specificity. Mol. Institute Pathol. 12, 187–201. doi: x.1111/j.1364-3703.2010.00659.ten
PubMed Abstract | CrossRef Full Text | Google Scholar
Xiao, S., Ellwood, S., Calis, O., Patrick, E., Li, T., Coleman, 1000., et al. (2001). Broad-spectrum mildew resistance in Arabidopsis thaliana mediated by RPW8. Scientific discipline 291, 118–120. doi: 10.1126/science.291.5501.118
PubMed Abstract | CrossRef Full Text | Google Scholar
Zhang, Y., Wang, 50., Xin, H., Li, D., Ma, C., Ding, 10., et al. (2013). Construction of a high-density genetic map for sesame based on large calibration marker evolution by specific length amplified fragment (SLAF) sequencing. BMC Constitute Biol. 13:141. doi: 10.1186/1471-2229-13-141
PubMed Abstruse | CrossRef Full Text | Google Scholar
Source: https://www.frontiersin.org/articles/10.3389/fpls.2015.00378/full
0 Response to "A Thaliana Defense Genes Agains Oomycetes"
Post a Comment